Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Effect of Different Stabilizers on Spherical Agglomerates of Montelukast Sodium by Quasi Emulsion Solvent Diffusion (QESD)
Authors: Hasiful Arabi J, Subramanian L, Rajesh M, Arun Kumar MS
DOI Link: https://doi.org/10.22214/ijraset.2023.55074
Certificate: View Certificate
Abstract
The present study was focused on the spherical crystallization of an asthmatic drug, Montelukast Sodium (MLS) using quasi emulsion solvent diffusion (QESD) technique in which distilled water was an external phase and the internal phase consisted of Ethanol which acts as good solvent and Ethyl acetate as a bridging liquid for recrystallization and agglomeration process. Apart from being poorly water soluble, MLS exhibits poor flow and compressibility. The spherical agglomeration was carried out in the presence of different stabilizers like acacia, PEG 4000, sodium lauryl sulphate, span 40 and tween 80. The prepared agglomerates were characterized in terms of production yield, drug content, solubility, in-vitro release profile, X-ray diffraction (XRD), and Fourier transform infra red spectroscopy (FT-IR).The optimized spherical agglomerates exhibited excellent physicochemical and micromeritic properties, solubility and dissolution rate when compared with pure drug .The XRD also revealed a characteristic decrease in crystallinity. The dissolution studies demonstrated a marked increase in the dissolution rate. FTIR study reveals there are no chemical changes in prepared recrystallized agglomerates. The considerable improvement in the dissolution rate of MLS from optimized crystal formulation was attributed to the wetting effect of polymers, decreased drug crystallinity, altered surface morphology and micronization. If this process can be scaled-up to manufacturing level, this technology has the potential to provide the directly compressed spherical agglomerates with improving the physicochemical and micromeritic properties.
Introduction
I. INTRODUCTION
The oral route remains the preferred route of drug administration due to its convenience, good patient compliance and low production costs. To be absorbed into the systemic circulation after oral delivery, a medication must first be dissolved in gastric juices. The dissolving process is the rate-controlling phase for hydrophobic medicines, determining the rate and degree of absorption. As a result, many hydrophobic medicines exhibit inconsistent and inadequate absorption in both animals and humans. As a result, poor solubility is one of the biggest obstacles to drug development today, with an estimated 40% of all newly discovered medications being weakly soluble or insoluble in water.1 Furthermore, due to their high lipophilicity, up to 50% of orally delivered medicinal compounds have formulation issues.2 As a result, extensive research has been performed to improve medication solubility and dissolution rates in order to boost the oral bioavailability of hydrophobic medicines. One method for improving dissolution is to reduce particle size and/or increase saturation solubility. Milling, a mechanical micronization technique, is one of the most prevalent methods for reducing particle size. Milling is a well-established technology that is relatively inexpensive, quick, and simple to scale up. However, milling has various drawbacks, the most significant of which is the restricted ability to control essential features of the finished particle such as size, shape, morphology, surface properties, and electrostatic charge. Furthermore, milling is a high-energy procedure that disrupts the drug's crystal structure, resulting in the presence of disordered or amorphous regions in the final product.3 Because these amorphous regions are thermodynamically unstable, they are prone to recrystallization during storage, especially in hot and humid circumstances.4
The modification of surface characteristics affects the milled product's saturation solubility, as well as its blending and flow properties, which has an impact on the formulation process. Furthermore, milled particles frequently exhibit aggregation and agglomeration, resulting in poor wettability and consequently poor dissolution5. An alternative to milling is to grow the particle from a solution to the appropriate size range under controlled conditions, such as spray drying, quasi emulsion solvent diffusion6 and supercritical fluid technologies.7,8
One of the advantages of these methods is the possibility of designing in certain beneficial characteristics such as enhancing dissolution rate by incorporating different polymers. The spherical crystallisation technique has been successfully applied these days to improve the micromeritic properties of drug substances.9 In the most common case, this technique is reputed to improve the wettability and dissolution rates of different drugs.10 Some drugs have also been recrystallized by the spherical agglomeration technique using polymeric materials to modify their release.11
There are two main methods for spherical crystallization: spherical agglomeration (SA method) and quasi-emulsion solvent diffusion (QESD method). In the SA method, a quasi-saturated solution of the drug in a solvent in which it is very soluble is poured into a poor solvent of the drug. Provided that the good and poor solvents are freely miscible and the interaction (binding force) between the solvents is stronger than the drug interaction with the good solvent, crystals precipitate immediately. A suitable amount of a third solvent, which is not miscible with the poor solvent and which preferentially wets the precipitated crystals, is added to the system while stirring. This third solvent, which is called a ‘bridging liquid’, can collect the crystals suspended in the system by forming liquid bridges between the crystals due to capillary negative pressure and interfacial tension between the interface of solid and liquid. When the interaction between the drug and the good solvent is stronger than that between the good and poor solvents, the good solvent drug solution is dispersed in the poor solvent, producing quasi-emulsion droplets, even if the solvents are normally miscible. This is due to an increase in the interfacial tension between good and poor solvents. Then the good solvent diffuses gradually out of the emulsion droplet into the poor solvent phase. The counterdiffusion of the poor solvent into the droplet induces the crystallization of the drug within the droplet due to the decreasing solubility of the drug in the droplet containing the poor solvent. This process is known as the quasi-emulsion solvent diffusion (QESD) process.12
The objective of this work was to evaluate the feasibility of the quasi-emulsion solvent diffusion technique to improve the solubility and dissolution characteristics of a poorly water-soluble drug, Montelukast Sodium. Moreover, Montelukast Sodium (a BCS class 2 drug) is poorly soluble in water. This work is focused primarily on evaluating the solvent diffusion technique on a lab scale for improving the solubility and dissolution characteristics of Montelukast Sodium. In addition, it also evaluates the effect of different stabilizers on solubility and dissolution rate. The in vitro release of the drug from the prepared agglomerated crystals was investigated and compared to that of the pure crystals. A microscopy study was used to study the surface characteristics of the granules. Furthermore, X-ray powder diffraction was utilized to investigate the crystallinity of the system.13
II. MATERIALS AND METHODS
A. Materials
Montelukast Sodium was obtained from Yarrow Chem Products, Mumbai. Ethanol and Ethyl acetate were obtained from Rankem Chemicals Pvt. Ltd. Acacia (Loba chemie Pvt. Ltd, Mumbai), Polyethylene glycol 4000 (Molychem, Mumbai), Sodium lauryl sulphate (Rankem Chemicals Pvt. Ltd.), Span 40 and Tween 80 were obtained from Thomas Baker Chemicals Pvt. Ltd, Mumbai.
B. Methods
All spherical agglomerates were prepared by the Quasi Emulsion Solvent Diffusion method. Spherical agglomerates were prepared with and without stabilizers by spherical crystallization technique. The codes of spherical agglomerated crystals of Montelukast sodium with different stabilizers was given in Table 1 and drug, stabilizers composition was given in Table 2. Montelukast sodium (1.0g) was dissolved in good solvent Ethanol (25.0ml).The bridging liquid Ethyl acetate (12.5ml) was added to it. The resulting solution was then poured drop wise in to the poor solvent distilled water (62.5ml) containing different stabilizer like acacia, Tween 80, Span 40, sodium lauryl sulphate, and polyethylene glycol (PEG 4000) with a stirring rate of 500 RPM using magnetic stirrer at room temperature. After agitating the system for 30 minutes, the prepared agglomerates were collected by filtration through whatman filter paper no.42. The same filtrate was used for subsequent washings of agglomerates. Then agglomerates were dried at 370C for 24 hours in a hot air oven.
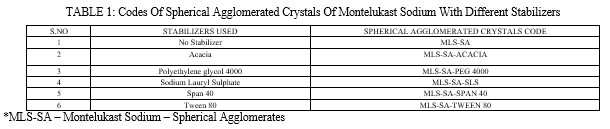
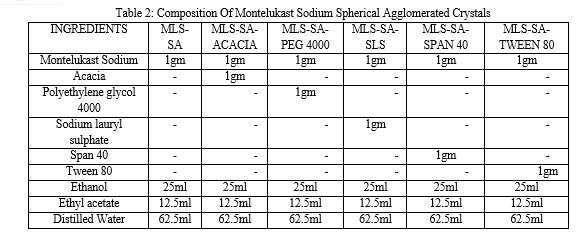
C. Particle Size Determination
Particle size determination was carried out using optical microscopy with a calibrated eye piece micrometer and stage micrometer by taking a small quantity of formulation on slide. About 50 spherical agglomerates size was measured individually, average was taken and their size range and mean diameter frequency was calculated. Average Particle size is calculated by the following formula,

D. Drug Content
The percentage drug content in spherical agglomerates was estimated by dissolving spherical agglomerates equivalent to 100 mg of Montelukast sodium in ethanol, mixing thoroughly by shaking, and making the volume up to the mark within a 6.8 pH phosphate buffer. The solution was filtered, the filtrate was diluted suitably with a 6.8 pH phosphate buffer, and absorbance was measured at 289 nm using a UV/visible spectrophotometer.
E. Percentage Yield
The percentage yield of spherical agglomerated crystals of Montelukast sodium prepared with the quasi emulsion solvent diffusion with three solvents method was determined by using the following equation:
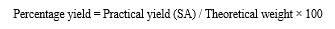
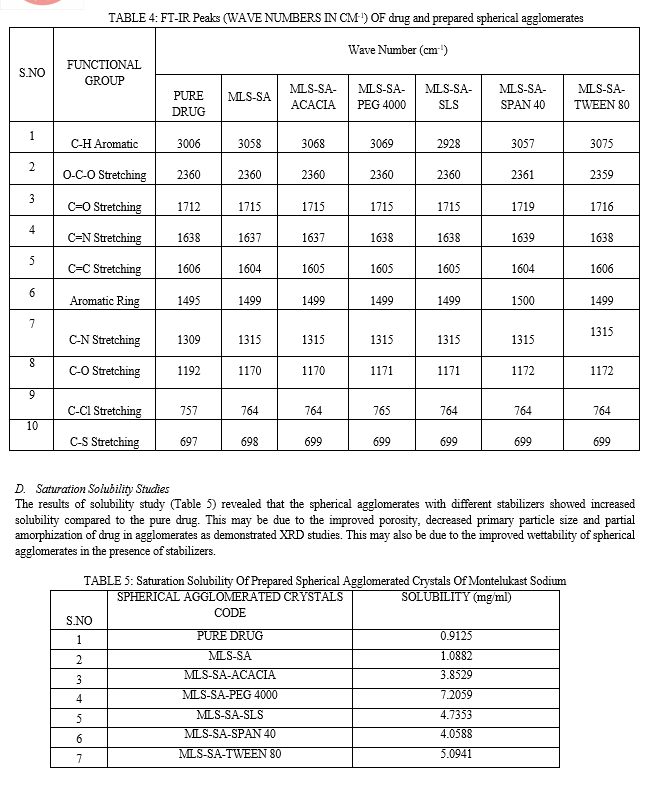
F. Saturation Solubility Studies
Montelukast sodium spherical agglomerate saturation solubility was determined in triplicate using the saturation solubility method. Excess amounts of SA were added to 10 ml of phosphate buffer with a pH of 6.8 in glass vials. The contents of the vials were vigorously mixed for 30 minutes before further solutions were mechanically shaken to equilibrate. Each vial's contents were centrifuged for 10 minutes at 2500 rpm after 72 hours. The supernatant of each vial was filtered through 0.45μ membrane filter, and the filtrate was diluted suitably with solvent separately. Monteluksat sodium concentration was determined using a double-beam UV-visible spectrophotometer (UV1800, Shimadzu, Japan) at 289 nm in comparison to a blank.
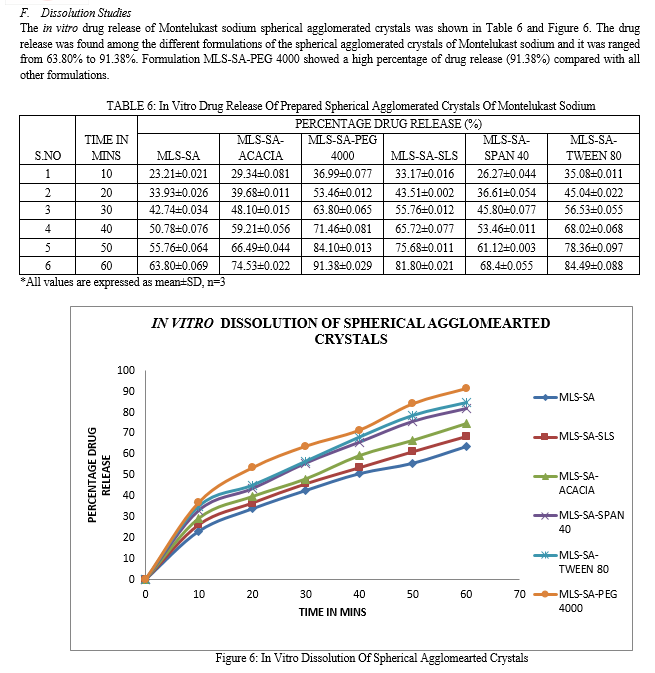
G. Dissolution Studies
In-vitro dissolution studies of spherical agglomerates were carried out for 60 minutes using the USP Dissolution test apparatus type I (Lab India DISSO 2000, eight stages) at 50 rpm. Spherical agglomerates equivalent to 50 mg of Montelukast sodium was used for dissolution study at 37±0.50oC in 900ml of 6.8 pH phosphate buffer as dissolution medium. Aliquot equal to 1 ml was withdrawn at regular time intervals (10, 20, 30, 40, 50, 60 min), an equal volume of fresh dissolution medium was replaced to maintain the sink condition and aliquots were measured at 289 nm UV/Visible spectrophotometer.
III. CHARACTERIZATION OF PREPARED SPHERICAL AGGLOMERATED CRYSTALS
A. Microscopical Study
The prepared agglomerated crystals of Montelukast sodium was spread on the glass slide using a glass rod. Formation of spherical agglomerated crystals of Montelukast sodium was confirmed by examining the prepared agglomerated crystals under an amscope microscope with the magnification power of 45X.
B. Melting Point Determination
Prepared agglomerated crystals of Montelukast sodium melting point was determined by placing a small amount of sample in a capillary tube that was closed at one end and placed in a melting point apparatus. The melting point was noted in triplicate and the average value was noted.
C. FT-IR (Fourier Transform Infrared Spectroscopy) Studies
FT-IR spectra of prepared spherical agglomerates were recorded on a Shimadzu FT-IR-8400 spectrophotometer (Shimadzu Corporation, Kyoto, Japan). The potassium bromide pellet method was employed, and background spectra were collected under identical conditions. Each spectrum was derived from single average scans collected in the region 400–4000 cm-1
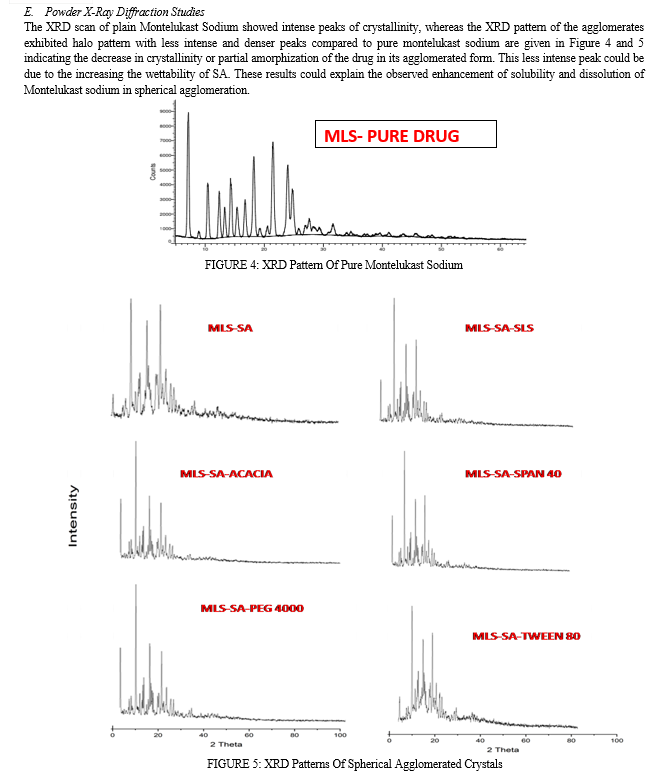
D. Powder X-Ray Diffraction Studies
Powder X-ray diffraction (PXRD) patterns were traced employing X-ray diffractometer for the samples using Ni filtered CuK(α) radiation (intensity ratio(α1/α2): 0.500), a voltage of 40 KV, a current of 30 mA and receiving slit of 0.2 inches. The samples were analyzed over 2 theta range of 5-70o with scanning step size of 0.020o (2 theta) and scan step time of one second.
IV. RESULTS AND DISCUSSION
All spherical agglomerates were obtained by the quasi- emulsion solvent diffusion method using distilled water as an external phase. The internal phase consisted of Ethanol which acts as good solvent and Ethyl acetate as bridging liquid for recrystallization and agglomeration process.
A. Microscopical Study
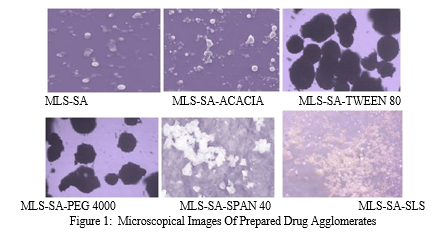
Formation of spherical agglomerated crystals was confirmed by examining the prepared drug agglomerates under an Amscope microscope with the magnification power of 45X and observes the morphology. The microscopy images are shown in Figure 1.
Microscopical images of developed drug agglomerates of Montelukast sodium demonstrate that tiny spheres of Montelukast sodium were detected in MLS-SA and MLS-SA-ACACIA. It also demonstrated that MLS-SA-TWEEN 80 and MLS-SA-PEG 4000 have distinct spheres of Montelukast sodium. Microscopical images of MLS-SA-SPAN 40 and MLS-SA-SLS indicate broken clump masses of Montelukast sodium drug spheres.
B. Percentage Yield, Drug Content And Particle Size
The percentage yield was found among the different formulations of the spherical agglomerated crystals and ranged from 88.49% to 94.24%. The percentage of drug content was found among the different formulations of the spherical agglomerated crystals and ranged from 91.2% to 98.2%. Formulation MLS-SA-PEG 4000 showed a high percentage of drug content (98.2%) and percentage yield (94.24%) compared with all other formulations.
The presence of stabilizers in spherical agglomerates influenced the particle size of resultant agglomerates. As the concentration of the increased, the size of the agglomerates increased. The presence of stabilizers on the particle surface increases particle–particle interaction, causing faster squeezing out of Ethanol to the Surface, resulting in increased particle size. The particle sizes were ranging from 107µm to 235µm in all the prepared spherical agglomerated crystals of Montelukast sodium. Formulation MLS-SA-PEG 4000 showed a large particle size (234.64) compared with all other formulations. This showed that all formulations have uniform particle size distribution.
The percentage yield, drug content and particle size of different formulations of the spherical agglomerated crystals are listed in Table 3.
TABLE 3: Percentage Yield, Drug Content And Particle Size Of Different Formulations Of The Spherical Agglomerated Crystals
|
S.NO |
SPHERICAL AGGLOMERATED CRYSTALS CODE |
Percentage Yield (%) |
Percentage Drug Content (%)
|
Average Particle Size (µm) |
|
1 |
MLS-SA |
89.34±0.086 |
93.6±0.112 |
107.69 |
|
2 |
MLS-SA-ACACIA |
89.96±0.113 |
91.2±0.045 |
112.12 |
|
3 |
MLS-SA-PEG 4000 |
94.24±0.132 |
98.2±0.094 |
234.64 |
|
4 |
MLS-SA-SLS |
91.41±0.049 |
96.2±0.098 |
179.46 |
|
5 |
MLS-SA-SPAN 40 |
88.49±0.045 |
89.8±0.065 |
163.55 |
|
6 |
MLS-SA-TWEEN 80 |
90.81±0.029 |
92.2±0.041 |
217.07 |
C. FT-IR (Fourier Transform Infrared Spectroscopy) Studies
The IR spectra of pure drug and all the samples are given in Figure 2 and 3. The prominent IR peaks (Wave numbers, cm-1) of drug and prepared spherical agglomerates are given in Table 4. The IR spectra of all the tested samples showed the prominent characterizing peaks of pure Montelukast Sodium which confirm that no chemical modification of the drug has been taken place.

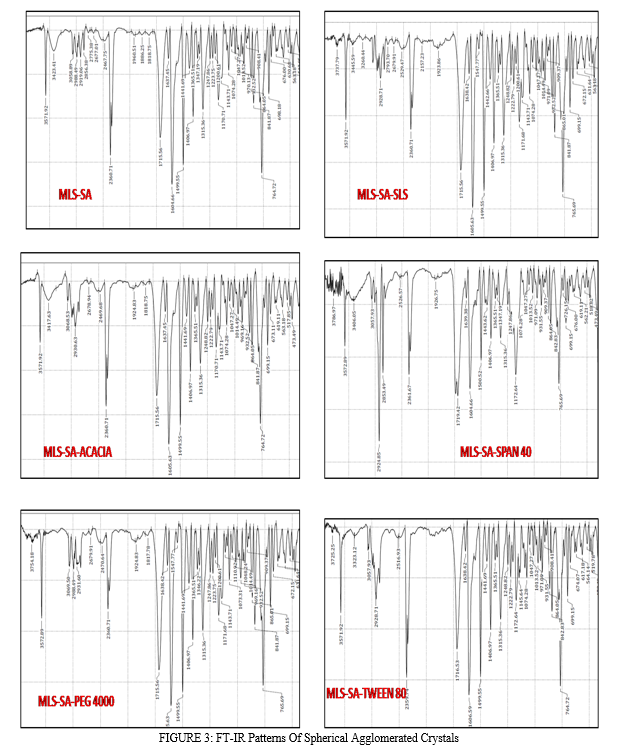
V. ACKNOWLEDGEMENTS
The authors wish to acknowledge and thanks to Dr.P.Solairaj, M.Pharm, Ph.D, Principal and Management of Sankaralingam Bhuvaneswari College of Pharmacy, Sivakasi, The authors also acknowledge and thanks to Instrumentation centres in ANJAC College, Sivakasi for FT-IR and VIT, Vellore for PXRD measurements. . In the same way, they would like to thank NIT Rourkela for their kind help in the Amscope microscopical photograph studies.
Conclusion
In this study prepared Montelukast sodium agglomerates exhibited excellent physicochemical and micromeritic properties, solubility, dissolution rate, flowability and packability when compared with pure drug as well as the physical mixture of drug with excipients . If this process can be scaled-up to manufacturing level, this technology has the potential to provide the directly compressed spherical agglomerates with improving the physicochemical and micromeritic properties.
References
[1] Naseem A., Olliff, C.J., Martini L.G., Lloyd A.W., Effects of plasma irradiation on the wettability and dissolution of compacts of griseofulvin,Int. J. Pharm., 2004,269, 443–450. [2] Gursoy R.N., Benita, S., Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drug, Biomed. Pharmacotherapy, 2004, 58,173–182. [3] Saleki-Gerhardt A., Ahlneck C., Zografi G.,Assessment of disorder in crystalline solids, Int. J.Pharm. 101, 144. [4] Ward G.H., Schultz, R.K., 1995. Processinduced crystallinity changes in albuterolsulfate and its effect on powder physical stability, Pharm. Res., 1994,12, 773–779. [5] Tur K.M., Ch’ng H.S., Baie, S., Use of bioadhesive polymer to improve the bioavailability of griseofulvin,Int. J. Pharm., 1997,148, 63–71. [6] Quintanar-Guerrero D., All´emann E., Fessi, H., Doelker E., Preparation techniques and mechanisms of formation of biodegradable nanoparticles from preformed polymers, Drug Dev. Ind. Pharm,1998,24, 1113–1128. [7] Hu J., Johnston K.P., Williams R.O., Spray freezing into liquid (SFL) particle engineering technology to enhance dissolution of poorly soluble drugs: organic solvent versus organic/aqueous co-solvent systems,Eur. J. Pharm. Sci., 2003,20, 295–303. [8] Moneghini M., Kikic I., Voinovich D., Perissutti B., Alessi P., Cortesi A., Princivalle F., Solinas D., Study of the solid state of carbamazepine after processing with gas anti-solvent technique, Eur. J. Pharm. Biopharm.,2003,56, 281–289. [9] Kawashima Y., Cui F., Takeuchi H., Niwa T.,Hino T.,Kiuchi K., Improvements in flowability and compressibility of pharmaceutical crystals for direct tabletting by spherical crystallization with a two-solvent system, Powder Technol,1994b,78, 151–157. [10] Kawashima Y., Handa T., Takeuchi H., Okumura M.,Katou H., Nagata O., Crystal modification of phenytoin with polyethylene glycol for improving mechanical strength, dissolution rate and bioavailability by a spherical crystallization technique, Chem. Pharm. Bull., 1986,34,3376–3383. [11] Akbuga J., Preparation and evaluation of controlled release furosemide microspheres by spherical mcrystallization, Int. J. Pharm., 1989, 53, 99–105.15. [12] Kawashima Y., Part 1. New processes. Application of spherical crystallization to particulate design of pharmaceuticals for direct tabletting, coating and new drug delivery system. In: Chulia, D., Deleuil, M., Pourcelot, Y. (Eds.), Powder Technology and Pharmaceutical Process.Elsevier, Amsterdam, 1994a.pp. 493–512. [13] Eldin, A. B., Shalaby, A. A., & El-Tohamy, M. (2011). Development and validation of a HPLC method for the determination of montelukast and its degradation products in pharmaceutical formulation using an experimental design. Acta Pharmaceutica Sciencia, 53(1), 45-56.
Copyright
Copyright © 2023 Hasiful Arabi J, Subramanian L, Rajesh M, Arun Kumar MS. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET55074
Publish Date : 2023-07-28
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

